FDA FSMA
FDA Food Safety Modernization Act
FSMA (Food Safety Modernization Act)
On January 4, 2011, the US government enacted the Food Safety Modernization Act (FSMA) for the purpose of protecting the safety of food supply and national health through prevention in advance. As a result, the FDA can focus on preventing food safety problems by establishing FSMA.
FSMA applies to facilities that manufacture, process, package or store food or feed. Also, companies that send food to the United States, such as producers, US importers, shippers, recipients, shippers, and transporters, must comply with FSMA. Therefore, according to FSMA, companies need to establish a food safety system that includes preventive management based on hazard analysis and prepare a documented food safety plan.

FSMA Accredited Third-Party Certification
IGC was accredited by the US FDA and IAS as a third party certification body for FDA FSMA, the 7th in the world and the first in Korea.

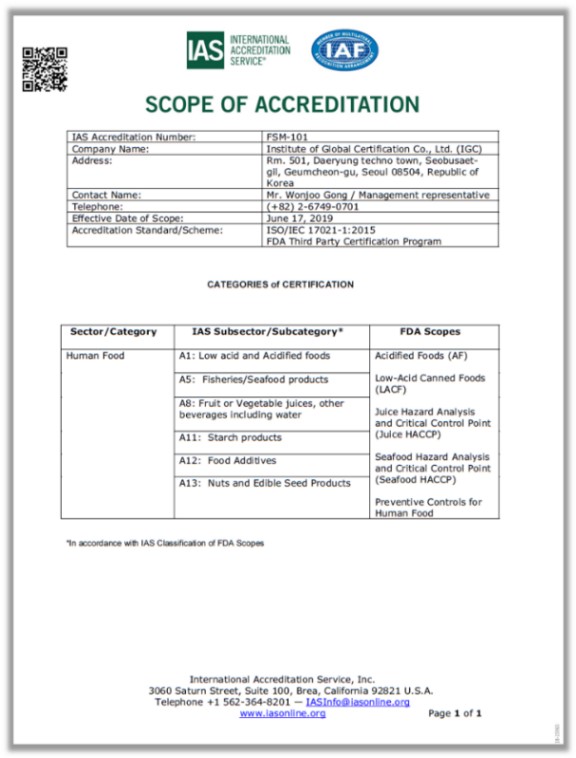
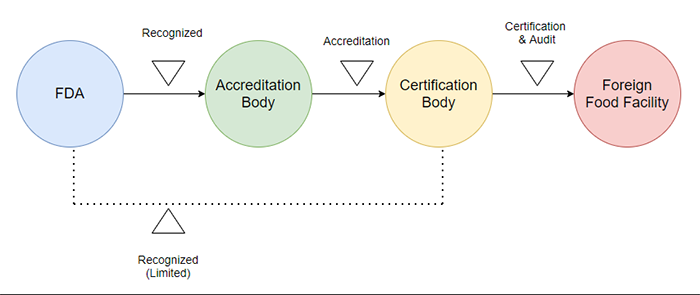
FSMA has a policy called Accredited Third-Party Certification Program. This program is designed to issue a certificate if the production facilities of foods produced outside of the United States comply with the requirements of FSMA by an authorized third party.
These certificates are used to qualify for the Voluntary Qualified Importer Program (VQIP) or to be used by the FDA to prevent the import of food that may be harmful to American clients.
1. Third-Party Certification Program procedure
Application for certification ⇒ Signing a contract ⇒ Payment of audit fees ⇒ Submission of documents ⇒ 1st stage audit ⇒ Certification review (1st stage) ⇒ 2nd stage audit ⇒ Certification review (2nd stage) ⇒ Certificate issuance ⇒ Surveillance audit ⇒ Recertification audit
2. Third-Party Certification Program
 FDA Food Safety Modernization Act Response Guidebook
FDA Food Safety Modernization Act Response Guidebook
3. Exempted from accredited third-party certification ★
- Alcoholic beverages manufactured at foreign facilities under certain circumstances - Specific meat, poultry and egg products supervised by the USDA at the time of import
-
-

-
FSMA Third Party Certification Preparation
Food facilities should establish and implement a food safety system that includes risk analysis and preventive management based on the risk. In addition, the food safety plan must be prepared in writing and reviewed a Preventive Control Qualified Individual (PCQI).
The food safety system shall establish a food safety plan that includes the following.- 1. Risk Analysis
- 2. Preventive management (process, food allergens, hygiene, supply chain and others, recovery plan)
- 3. Monitoring and operation of preventive management
- 4. Corrective action and correction
- 5. Verification
In the food safety plan, a recovery plan should be established so that products that are problematic for food safety can be recalled. In response, IGC also provides training courses for PCQI for the organization which related to the food that are exporting or planning to the US market.
-
-
-

-
The importance of FSMA Third Party Certification
FSMA focuses on prevention of food safety, which places new requirements from food manufacturers, facilities and importers in the United States and outside the United States. Therefore, selling and distributing products in the US market requires compliance with new requirements. In order to facilitate food exports to the U.S market in response to FSMA, organization that export food to the U.S. need careful preparation.
-
-
-
List of FSMA 3rd party certified organizations
Certificate No. 23-FSMA-0008 23-FSMA-0009 Eligible Entity BUSAN EOMUK CO., LTD. PELICANA & FOOD CORP. Facility Address 69-25, Junam-ro, Yangsan-si, Gyeongsangnam-do, Republic of Korea 79, Wangdae-ro, Duma-myeon, Gyeryong-si, Chungcheongnam-do, Republic of Korea Code Human Food Category 5, 11 Regulation: CFR 21, Chapter I, Subchapter B, parts # 117, 123 Human Food Category 12 Regulation: CFR 21, Chapter I, Subchapter B, parts # 117 Duration 2023-07-25 ~ 2024-07-24 2023-10-11 ~ 2024-10-10 Scope Manufacture of fish cake, processed sea food products and easy-to-cook food Manufacture of natural seasoning foods, mixed prepared seasonings and chicken sauces Payment Date 2023-06-30 2023-08-07 Audit Date ST1: 2023-06-14
ST2: 2023-06-21ST1: 2023-08-22
ST2: 2023-09-01
-
-
-

-
IGC’s Competency
IGC was accredited by the US FDA and IAS as the FSMA Third Party Certification Body, the 7th in the world and the first in Korea.
IGC has extensive knowledge of food safety regulations as it operates a food laboratory and conduct various food-related certifications.
IGC provides fast and accurate services for our clients, helping clients make the right choices and demonstrate due diligence with FSMA compliance services.
IGC is a leading provider of the FSMA Third Party Certification Body and provides fast and accurate certification services.
-
Related Services from IGC
